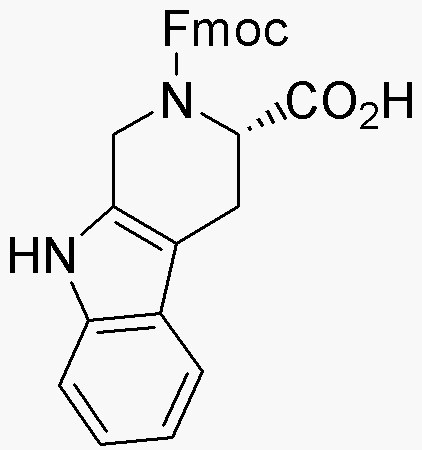
Fmoc-L-1,2,3,4-tetrahydronorharman-3-carboxylic acid is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a protective group in solid-phase peptide synthesis, allowing for the selective modification of amino acids without interfering with other functional groups.
- Drug Development: It is used in the design of novel pharmaceuticals, particularly in the development of compounds targeting neurological disorders, due to its structural similarity to biologically active molecules.
- Bioconjugation: The chemical facilitates bioconjugation processes, enabling the attachment of biomolecules to therapeutic agents, which enhances drug delivery and efficacy.
- Research in Neuroscience: Its unique structure makes it a valuable tool in neuroscience research, helping scientists explore the mechanisms of neuroactive compounds and their potential therapeutic effects.
- Analytical Chemistry: The compound is employed in analytical methods to study interactions between biomolecules, providing insights into molecular behavior and aiding in the development of new analytical techniques.
Información general
Propiedades
Seguridad y normativas
Aplicaciones
Fmoc-L-1,2,3,4-tetrahydronorharman-3-carboxylic acid is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a protective group in solid-phase peptide synthesis, allowing for the selective modification of amino acids without interfering with other functional groups.
- Drug Development: It is used in the design of novel pharmaceuticals, particularly in the development of compounds targeting neurological disorders, due to its structural similarity to biologically active molecules.
- Bioconjugation: The chemical facilitates bioconjugation processes, enabling the attachment of biomolecules to therapeutic agents, which enhances drug delivery and efficacy.
- Research in Neuroscience: Its unique structure makes it a valuable tool in neuroscience research, helping scientists explore the mechanisms of neuroactive compounds and their potential therapeutic effects.
- Analytical Chemistry: The compound is employed in analytical methods to study interactions between biomolecules, providing insights into molecular behavior and aiding in the development of new analytical techniques.
Documentos
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
Especificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
Certificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.
Número de catálogo
Número de lote/lote
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
DownloadEspecificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
DownloadCertificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.