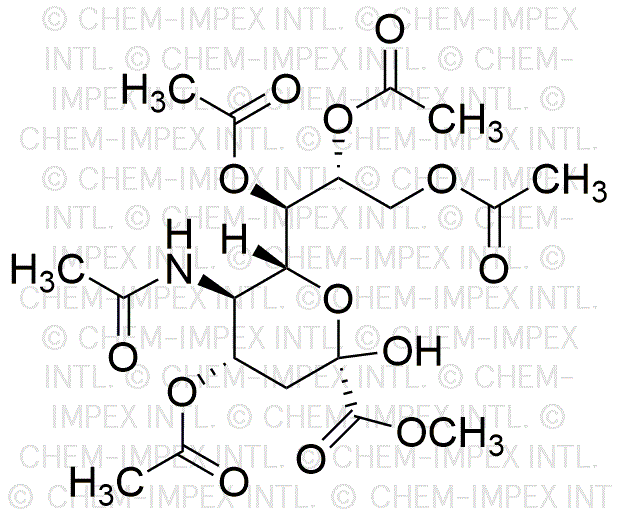
Methyl 4,7,8,9-tetra-O-acetyl-N-acetylneuraminate is widely utilized in research focused on:
- Biochemical Research: This compound serves as a valuable substrate in studies investigating sialic acid metabolism and its role in cellular interactions, particularly in the context of viral infections and immune responses.
- Vaccine Development: It is used in the formulation of vaccines, where its sialic acid derivatives can enhance the immunogenicity of antigens, improving vaccine efficacy against various pathogens.
- Drug Design: Researchers leverage its structural properties to design inhibitors targeting sialidases, which are enzymes involved in the pathogenicity of several viruses and bacteria.
- Glycobiology: This compound aids in the study of glycoproteins and glycolipids, helping scientists understand their functions in cell signaling and recognition processes.
- Diagnostic Applications: It is utilized in the development of diagnostic assays for detecting sialic acid-related diseases, providing insights into conditions such as cancer and neurodegenerative disorders.
General Information
Properties
Safety and Regulations
Applications
Methyl 4,7,8,9-tetra-O-acetyl-N-acetylneuraminate is widely utilized in research focused on:
- Biochemical Research: This compound serves as a valuable substrate in studies investigating sialic acid metabolism and its role in cellular interactions, particularly in the context of viral infections and immune responses.
- Vaccine Development: It is used in the formulation of vaccines, where its sialic acid derivatives can enhance the immunogenicity of antigens, improving vaccine efficacy against various pathogens.
- Drug Design: Researchers leverage its structural properties to design inhibitors targeting sialidases, which are enzymes involved in the pathogenicity of several viruses and bacteria.
- Glycobiology: This compound aids in the study of glycoproteins and glycolipids, helping scientists understand their functions in cell signaling and recognition processes.
- Diagnostic Applications: It is utilized in the development of diagnostic assays for detecting sialic acid-related diseases, providing insights into conditions such as cancer and neurodegenerative disorders.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Número de catálogo
Número de lote/lote
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.