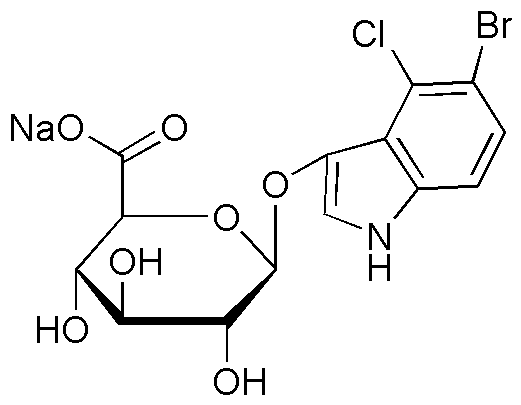
5-Bromo-4-chloro-3-indolyl-b-D-glucuronide sodium salt is widely utilized in research focused on:
- Histochemical Staining: This compound is commonly used as a substrate in histochemical assays to visualize β-glucuronidase activity in tissue samples, aiding researchers in studying enzyme localization and activity.
- Drug Metabolism Studies: It serves as a model substrate for investigating the metabolism of glucuronidated drugs, helping pharmaceutical researchers understand drug interactions and clearance rates.
- Cellular Assays: This chemical is employed in cellular assays to monitor cellular processes, such as apoptosis and proliferation, providing valuable insights into cell behavior in response to various treatments.
- Biochemical Research: It is used in biochemical studies to explore the role of glucuronidation in detoxification pathways, enhancing our understanding of metabolic processes in different organisms.
- Environmental Monitoring: The compound can be utilized in environmental studies to assess the impact of pollutants that undergo glucuronidation, contributing to ecological research and sustainability efforts.
Información general
Propiedades
Seguridad y normativas
Aplicaciones
5-Bromo-4-chloro-3-indolyl-b-D-glucuronide sodium salt is widely utilized in research focused on:
- Histochemical Staining: This compound is commonly used as a substrate in histochemical assays to visualize β-glucuronidase activity in tissue samples, aiding researchers in studying enzyme localization and activity.
- Drug Metabolism Studies: It serves as a model substrate for investigating the metabolism of glucuronidated drugs, helping pharmaceutical researchers understand drug interactions and clearance rates.
- Cellular Assays: This chemical is employed in cellular assays to monitor cellular processes, such as apoptosis and proliferation, providing valuable insights into cell behavior in response to various treatments.
- Biochemical Research: It is used in biochemical studies to explore the role of glucuronidation in detoxification pathways, enhancing our understanding of metabolic processes in different organisms.
- Environmental Monitoring: The compound can be utilized in environmental studies to assess the impact of pollutants that undergo glucuronidation, contributing to ecological research and sustainability efforts.
Documentos
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
Especificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
Certificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.
Número de catálogo
Número de lote/lote
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
DownloadEspecificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
DownloadCertificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.